Arrange the Following Bonds by Increasing Bond Polarity.
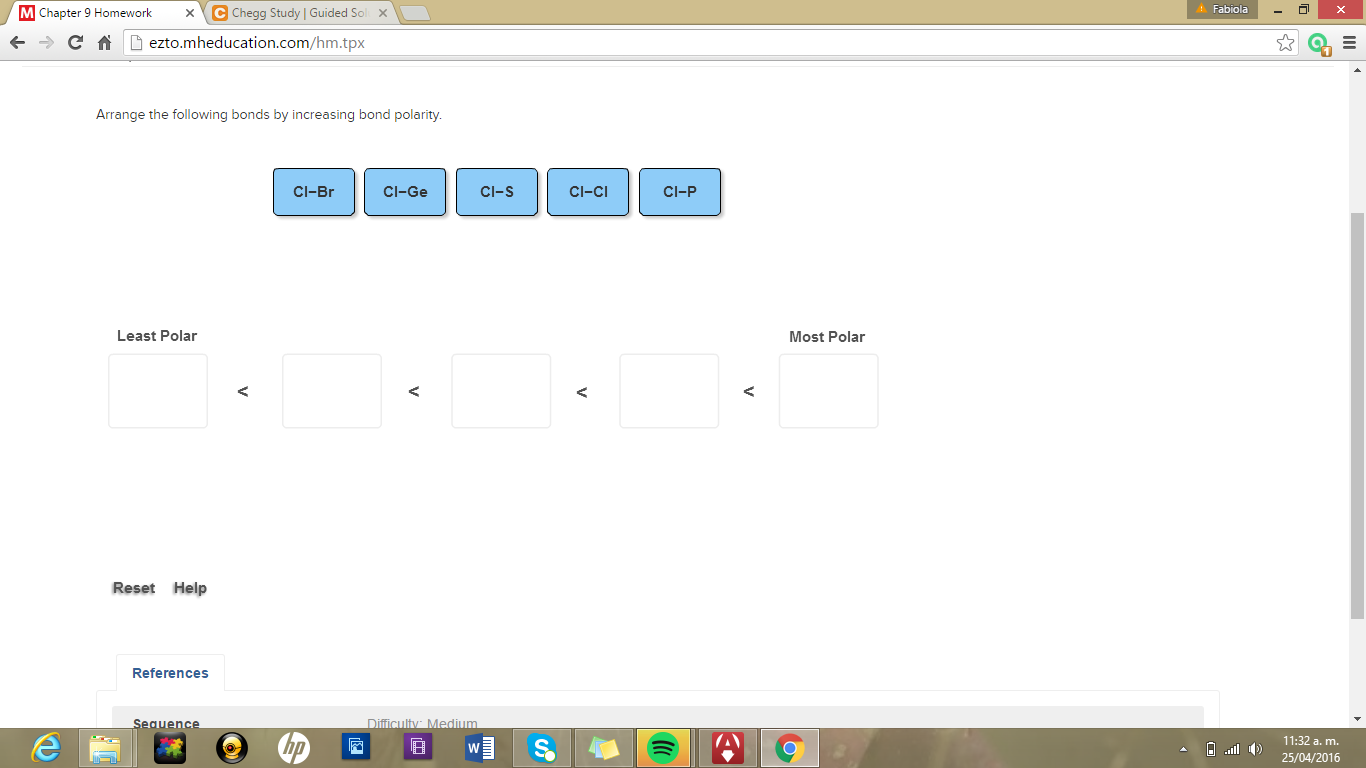
Cl-S Cl-Si Cl-P Cl-Cl. Video Player is loading.
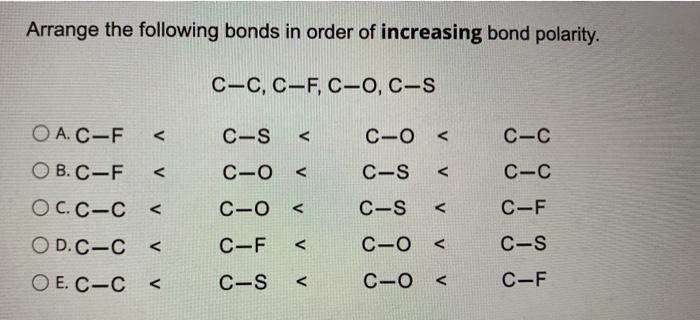
Solved Arrange The Following Bonds In Order Of Increasing Chegg Com
Which of the following would be a polar molecule.
. Arrange the following bonds in order of increasing polarity. Then arrange the bonds from smallest E value to greatest _E value. F-F F-C F-O F-N.
There will always be a small amount of electron sharing. Question 8 Arrange the following bonds in order of increasing polarity. P - H H - O N - H H - F.
Science Chemistry QA Library Arrange the bonds in each of the following sets in orderof increasing polarity. C HN H O H the hydrogen will have a slight positive charge while C N and O atoms will have a slight negative charge are they are more electronegative than the hydrogen. Both polar bonds and an unsymmetrical arrangement.
Use the appropriate symbol to separate substances in the list aAs-Br Ca-Br Ga-Br bBe-F Li-F N-F cCa-Br. Then designate the positive and negative atoms using the symbols δ and δ. If we have a proton and electron separated by.
Question 8 Arrange the following bonds in order of increasing polarity O H C H F from CHEM 1411 at Trinity Valley Community College. Cl2 CaCl2 CsCl PCl3 CCl4. Thus greater is the electronegativity difference greater the polarity of bond.
The shape of a BF3 molecule is. A mathrm HCl b mathrm PH_ 3 c mathrm H_ 2 mathrm O d mathrm CF_ 4 Problem. O-H C-H F-H H-H A O-H B C-H.
Ammonia N H 3 and phosphorus trihyd. CH CN CO NH OH SH. A CF OF BeFb OCl SBr CP.
The anion in an ionic compound whose name ends in ide is. Arrange the following bonds in order of increasing bond polarity. Arrange the bonds in each of the following sets in order of increasing polarity.
This means that in order of increasing polarity you can arrange these bonds like this colorgreenCl-O C-O P-O Alternatively you can consult the actual electronegativity values and confirm that weve got the order right. Solution Verified by Toppr Correct option is B Solution- B OFNFBeFMgF As we know that the polarity of bond is directly proportional to the electronegativity difference. A H Cl b C- O c N-F d Si- I and e O O.
Trinity Valley Community College. Arrange the following molecules by increasing bond polarity. Using the electronegativity values in Table A2 arrange the following covalent bondsall commonly found in amino acidsin order of increasing polarity.
Cl-S Cl-P Cl-Si Cl-Cl. First calculate the electronegativity difference E between atoms in each bond. BaCl BrCl BCl BrCl ClCl.
Arrange the following bonds in order of increasing polarity. As dipole formation is a characteristic of bond polarity the molecules exhibiting dipoles are more precisely called Polar covalent molecules. Use - to represent a single bond.
Cl-Cl Cl-P Cl-Si Cl-S. Since we know the electro negativity of an atoms gets increased while moving across the period and due to which the. Here in the given polar covalent bonds.
Where the final bond ClClis of. Cl-S Cl-P Cl-Si Cl-Cl. In simple words a bond polarity is a scientific tool that gives us an idea about the nature of the bonds and the type of bonding they will undergo to form compounds.
Cl-Cl Cl-S Cl-P Cl-Si. A CF OF BeFb OCl SBr CP. A H- Cl bond E 21 30 09 b C- O bond E 25 35 10.
In practice no bond is totally ionic. Where Q the magnitude of the charge on each end of the dipole and r is the distance between the positive and negative charges. H-H O-H Cl-H S-H and F-H.
Among the bonds listed therefore the BaCl bond corresponds to the largest difference in electronegativity ie to the most nearly ionic bond. The order of bond polarity is thus. Cl-Si Cl-S Cl-Cl Cl-P.
Arrange the given bonds in increasing order of polarity. Arrange the following bonds in order of increasing polarity. Arrange the following bonds in order of increasing polarity.
P - H H - O N - H H - F. The bigger the difference between oxygen and the other atoms electronegativity values the more polar the bond will be. Order the following bonds according to polarity.
Arrange the given bonds in increasing order of polarity. SOLVEDArrange the following molecules in order of the increasing polarity of their bonds. C CS BF NO Arrange the bonds in each of the following sets in orderof increasing polarity.
Hence the correct increasing order of polarity of bonds is- OFNFBeFMgF. C CS BF NO. The polarity of these bonds increases as the absolute value of the electronegativity.

Solved Arrange The Following Bonds By Increasing Bond Chegg Com
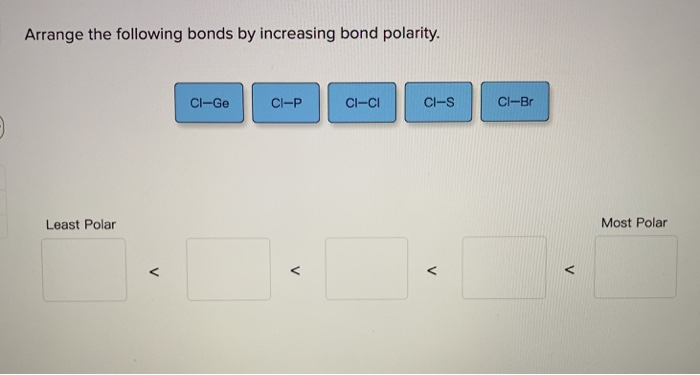
Solved Arrange The Following Bonds By Increasing Bond Chegg Com

Solved Question 13 Arrange The Following Bonds In Order Of Chegg Com
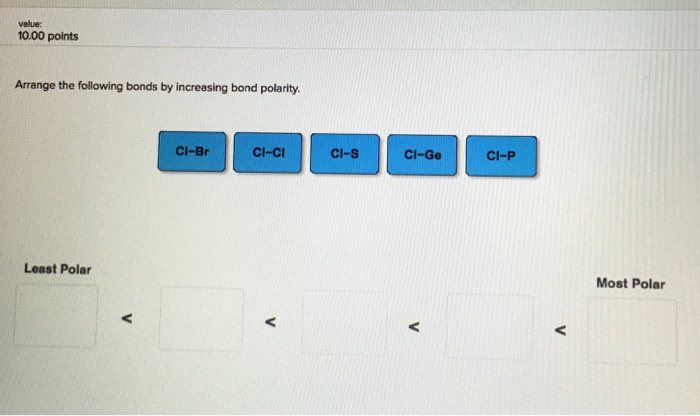
Solved Value 10 00 Points Arrange The Following Bonds By Chegg Com
Comments
Post a Comment